
Please tell us about colonoscopy challenges prior to the introduction of AI technology to the field.
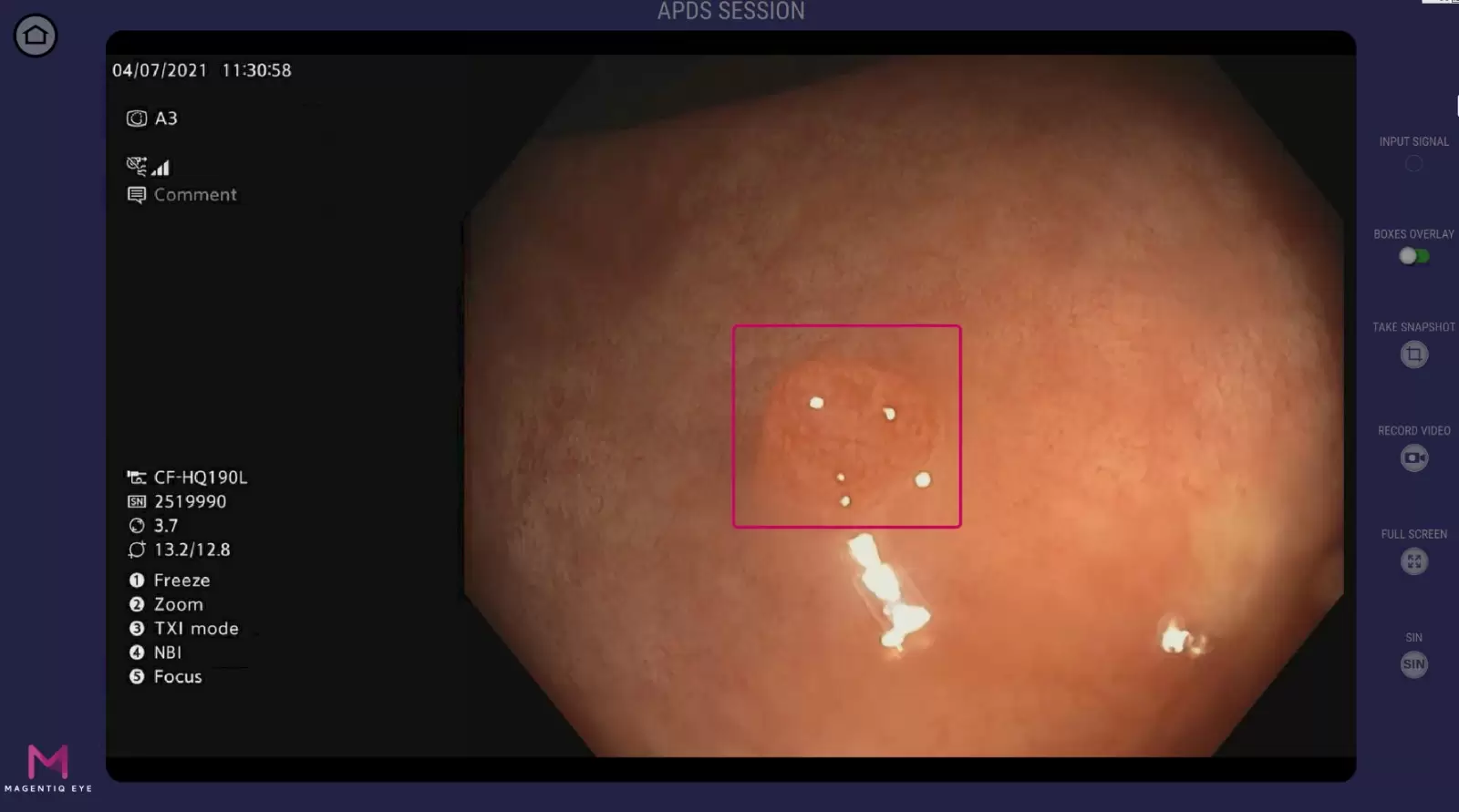
Prior to 2018 when we first met MAGENTIQ EYE, it was clear that as a field we needed to improve the quality of polyp detection and bring down miss rates. The Post Coloscopy Colon Cancer diagnosis, where a patient has colon cancer relatively shortly after undergoing colonoscopy, was at very high rates and we understood that a great number of these cases was a result of polyps being missed. There was a real need out there to improve detection, and when presented with AI and MAGENTIQ COLO it was clear that this was a technology that could really address that.
The second issue was quality: quality assurance and quality control. There was a lot of interest around questions like how to measure the quality of endoscopy? how can we make sure we’re detecting polyps that should be detected? and how can physicians compare performance with others doing coloscopy? Discussions on these issues took place everywhere and there was a tremendous amount of interest in technology that could help address them.
What unique value did you see in MAGENTIQ COLO then, and what is it today, compared to other solutions you tested?
MAGENTIQ EYE was the first AI solution we were presented with; this was before even the big companies released their AI assisted products. We were very impressed by the magnitude of their machine learning study which gave us the confidence that AI was in fact capable of detecting polyps, and could easily be integrated into the normal work flow of an endoscopy unit. The decision to enter MAGENTIQ EYE’s trial was obvious.
The clinical study conducted by MAGENTIQ EYE which Hadassah took part in, is to the best of my knowledge, the largest randomized, blinded, international, multicenter control trial ever done on artificial intelligence and polyp detection. The study confirms that using the system there’s a 37% relative increase in Adenoma Per Colonoscopy (APC), a 26% relative increase in ADR, and a 48% relative decrease in AMR. These results set a new standards of colonoscopy performance.
At present, MAGENTIQ EYE is gearing up to releasing the next version of MAGENTIQ COLO platform which improves detection even further, and adds two important features: estimated polyp size and histology (type of polyp). The platform will continue to develop and users can expect to see more and more features in the future.
Since we started using MAGENTIQ COLO, I’ve tested most of the systems available in the market, and I can honestly say this is the best and most robust system out there, hands down. Some may think I’m biased, but when the results are there, they speak for themselves.
Recently, MAGENTIQ COLO received FDA clearance. What does this milestone mean for Hadassah Medical Center and other medical centers using the device?
FDA clearance is definitely a milestone as it opens the door to market the system in the USA, in addition to Europe and Israel.
Hadassah Medical Center uses the system in the majority of colonoscopies we perform, and as a research partner, we also supply data (with patient consent) which is used to develop additional features. With only a handful of companies in the field holding this clearance, it provides additional validation that MAGENTIQ COLO is up there competing with the best.
Is AI the future of colonoscopy?
I believe it is. First and foremost, it addresses the shortcomings of standard colonoscopy, and assures that all colonoscopies are performed at the highest standard. We’re also seeing a growing number of patients asking for it, because they want that extra safety net which AI provides. For the medical community, AI allows residents to develop their skills quickly, and bridges the experience gap between physicians with less colonoscopy experience to top tier experts. This is especially important in places where the demand for colonoscopy, and shortage in experts requires clinics to provide services around the clock.

